MEAT
names
pyrotechne
tunic
chronic
alchemistry
crude
thrown fire
in law
love's fire
illuminated
tourist
texts
sources
versions
Man came here by an intolerable way. When man is reduced to so much fat for soap, superphosphate for soil, filings and shoes for sale, he has, to begin again, one answer, one point of resistance only to such fragmentation, one organized ground, a ground he comes to by a way the precise contrary of the cross, of spirit in the old sense, in old mouths. It is his own physiology he is forced to arrive at. And the way--the way of the beast, of man and the Beast.
It is his body that is his answer, his body intact and fought for, the absolute of his organism in its simplest terms, this structure evolved by nature, repeated in each act of birth, the animal: man; the house he is, this house that moves, breathes, acts, this house where his life is, where he dwells against the enemy, against the beast.
Or the fraud. This organism now our citadel never was cathedral, draughty tenement of soul, was what it is: ground, stone, wall, cannon, tower. In this intricate structure are we based, now more certainly than ever (besieged, overthrown), for its power is bone muscle nerve blood brain a man, its fragile mortal force its old eternity, resistance.
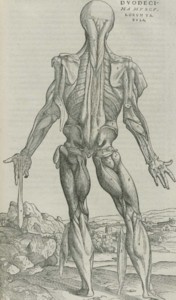
Charles Olson, "The Resistance"
for Jean Riboud (1953),
Collected Prose 174
"In the general effervescence of life, the tiger is a point of extreme incandescence. And this incandescence did in fact burn first in the remote depths of the sky, in the sun's consumption.
Eating brings death, but in an accidental form. Of all conceivable luxuries, death, in its fatal and inexorable form, is undoubtedly the most costloy. The fragility, the complexity, of the animal body already exhibits its luxurious quality, but this fragility and luxury culminate in death.''
Georges Bataille, The Accursed Share, vol. 1 p. 34.
An eminent English chemist, Dr Charles Henry Maye, set out to establish in a precise manner what man is made of and what is its chemical value. This is the result of his learned researches:
The bodily fat of a normally constituted man would suffice to manufacture seven cakes of toilet-soap. Enough iron is found in the organism to make a medium-sized nail, and sugar to sweet a cup of coffee. The phosphorus would provide 2,200 matches. The magnesium would furnish the light needed to take a photograph. In addition, a little potassium and sulphur, but in an unusable quantity.
These different raw materials, costed at current prices, represent an approximate sum of 25 francs.
Journal des Debats, 13 Aug 1929; cited in 'Critical Dictionary', Georges Bataillle, Michel Leiris, Marcel Griaule, Carl Einstein and Robert Desnos, eds., Encyclopaedia Acephalica.
photo © Mike Perkowitz
PHOSPHOROUS
The amount of phosphorus per kg in the body:
Teeth: 130g
Bone: 110g
Brain: 3.4g (avg)
Liver: 2.7g
Muscle: 1.8g
The high concentration of phosphorus in the brain led Dr Kramer to prescribe it as a remedy for epilepsy and melancholia (1730).
"De Lens affirms that the has been told by those who were in the habit of handling phosphorus frequently that they suffered from venereal excitation." Dr Ashburton Thompson, Free Phosphorous in Medicine (1874).
Johann Lincke, proprietor of the Golden Lion pharmacy in Leipzig, sold "Kunckel's pills", 200 mg of phosphorus coated in gold or silver. Phosphorus was also prescribed as a tincture, dissolved in edible oil, or rubbed on the skin as a lineament.
Phosphorus was recommended as a treatment for nervous disorders, weight loss, indigestion, etc. Leroy advocated drinking phosphorus water as a tonic. Due to its ability to change bone structure, it was also recommended to treat rickets and fractures, and still available in the UK as an over-the-counter remedy in the 1950s. Sanatogen Tonic Wine, still sold, consists of 0.62% sodium glycerophosphate.

In the 1960s, Procter and Gamble investigated other uses for the phosphate water-softening chemicals in their detergents. This led to the discovery of biophosphonates, which are used to treat bone conditions such as Paget's disease and osteoporosis. They include: pamidronate, risedronate, ibandronate, and Zoldronate.
Metrifonate was developed in the 1950s as an insecticide. It is now used to treat Alzheimer's: it reduces the activity of the ACHhE enzyme in the brain.
SARIN
The disruption of AChE also has military uses. Sarin, a phosphorus-fluorine compound first made by Gerhard Schrader's team at IG Farben in 1935, is an organophosphate nerve gas. It attacks the central nervous system (resulting in giddiness, anxiety, headache, confusion, convulsions and difficulty in breathing), disrupts the regulation of the glands (resulting in profuse phlegm, sweating and salivation), and causes erratic muscle response (resulting in stomach cramps, irregular heartbeat, incontinence and and miosis - the closing of the iris of the eye.)
The effects of nerve gas were tested on volunteers at the Chemical Defence Experimental Establishment at Porton Down, Wiltshire, on 6 May 1953; Lance Corporal Ronald Maddison was killed.
Sarin was used in 1988 on Kurdish villagers in northern Iraq, and on 19 April 1995 by the Aum Shinrikyo doomsday sect in Tokyo, Japan.
The antidotes for nerve gas are atropine, oxime and diazepam (Valium), dispensed by a "Combo pen" which can be inserted through the clothes.
source: Elmsley pp. 166-7
results in painful chemical burn injuries. burn typically appears as a necrotic area with a yellowish color and characteristic garliclike odor.
highly lipid soluble. rapid dermal penetration once particles are embedded under the skin. delayed wound healing.
incandescent particles of WP may produce extensive burns. a firm eschar is produced and is surrounded by vesiculation. burns usually are multiple, deep, and variable in size.
WP tends to burn its way through the skin and, even days after the original injury, spontaneously igniting particles may be found deep in the wound. Frequently localized on arm, hand, thigh, lower leg and head. Spreads rapidly through the fatty tissue underlying the dermis.
The severe full thickness chemical burn may appear deceptively superficial with only a greyish-brown discolouration of intact skin during the first few days.
Complications include: contracture of joints; ectropion; chondritis of ears; gangrene; cataract; pneumonia; ankylosis; gastrointestinal bleeidng; corneal ulcer; cullulitis or lymphangitis; cutaneous abscess; septicaemia; renal failure; osteomyelitis; jaundice; urinary tract infaction.
Anoxia of the brain, indicated by restlessness, toxic delirium, toxic psychosis, hallucinations or maniacal manifestations, usually leads to death in patients with phosphorus poisoning.
(an axis running to the Salpêtrière hospital for the insane in Paris? saltpetre, used in gunpowder, manufactured at this old arsenal...)
the solid in the eye produces severe injury. White phosphorus fume can cause blepharospasm, photophobia, and lacrimation.
particles continue to burn unless deprived of atmospheric oxygen. could burn right down to the bone.
carry a greater risk of mortality than other forms of burns due to the absorption of phosphorus into the body through the burned area, resulting in liver, heart and kidney damage, and in some cases multi-organ failure. The Agency for Toxic Substances and Disease Registry has set an acute inhalation Minimum Risk Level (MRL) for white phosphorus smoke of 0.02mg/m³, the same as fuel oil fumes. (By contrast, the chemical weapon mustard gas is 30 times more potent: 0.0007 mg/m³.)
casualties from WP smoke have not occurred in combat operations. with intense exposures, a very explosive cough may occur, which renders gas mask adjustment difficult. spontaneous recovery is rapid.
take off the contaminated clothing quickly before the WP burns through to the skin. plunge skin or clothing affected by phosphorus in cold water or moisten strongly to extinguish or prevent fire. rinse affected skin areas with cold sodium bicarbonate solution or with cold water. remove visible phosphorus (preferably under water) with squared object (knife-back etc.) or tweezers. do not touch phosphorus with fingers! throw into water or allow to bum in suitable location. cover burns with moist, saline-soaked dressing to prevent renewed inflammation or reignition of the phosphorus by contact with air.
wash skin with a 0.5-2.0% copper sulphate solution or a copper sulphate impregnated pad. dark coloured deposits may be removed with forceps. prevent prolonged contact of any copper sulphate preparations with the tissues by prompt, copious flushing with water or saline.
A Wood’s lamp in a darkened room may help to identify remaining luminescent particles.
Left to herself, the serpent now began
To change; her elfin blood in madness ran,
Her mouth foam’d, and the grass, therewith besprent,
Wither’d at dew so sweet and virulent;
Her eyes in torture fix’d, and anguish drear,
Hot, glaz’d, and wide, with lid-lashes all sear,
Flash’d phosphor & sharp sparks, without one cooling tear.
The colours all inflam’d throughout her train,
She writh’d about, convuls’d with scarlet pain:
A deep volcanian yellow took the place
Of all her milder-mooned body’s grace;
And, as the lava ravishes the mead,
Spoilt all her silver mail, and golden brede;
Made gloom of all her frecklings, streaks and bars,
Eclips’d her crescents, and lick’d up her stars:
So that, in moments few, she was undrest
Of all her sapphires, greens, and amethyst,
And rubious-argent: of all these bereft,
Nothing but pain and ugliness were left.
"The splinter in your eye is the best magnifying glass."
John Keats, "Lamia"
Phosphorus causes yellowing of the skin and hair loss and phossy jaw, a form of bone cancer. the whole side of the face turns green and then black, discharging foul-smelling pus and finally death.
the first known case of phossy jaw was that of Marie Jankovitz in Vienna in 1838.
on average, phossy jaw emerged after five years' exposure to phosphorus.
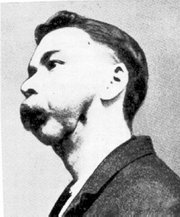
In the late 1800s, the condition was more prevalent in the UK than in other countries, because the match-making industry was not mechanized, and many workers were directly exposed to phosphorus fumes.
early signs of systemic intoxication by phosphorus are abdominal pain, jaundice, and a garlic odor of the breath; prolonged intake may cause anemia, as well as cachexia and necrosis of bone, involving typically the maxilla and mandible (phossy jaw). necrosis of bones. a hepatotoxin.
presenting complaints of overexposed workers may be toothache and excessive salivation.
a dull red appearance of the oral mucosa. one or more teeth may loosen, with subsequent pain and swelling of the jaw; healing may be delayed following extractions; with necrosis of bone, a sequestrum may develop with sinus tract formation.
In a series of 10 cases, the shortest period of exposure to phosphorus fume (concentrations not measured) that led to bone necrosis was 10 months (two cases), and the longest period of exposure was 18 years.
Phossy jaw must be differentiated from other forms of osteomyelitis. With phossy jaw, a sequestrum forms in the bone and is released from weeks to months later; the sequestra are light in weight, yellow to brown, osteoporotic, and decalcified, whereas sequestra from acute staphylococcal osteomyelitis are sharp, white spicules of bone, dense and well calcified. In acute staphylococcal osteomyelitis, the radiographic picture changes rapidly and closely follows the clinical course, but with phossy jaw the diagnosis sometimes is clinically obvious before radiological changes are discernible. It is good dental practice to take routine X-ray films of jaws, but experience indicates that necrosis can occur in the absence of any pathology that is visible on the roentgenogram.
Bryant & May had an on-sight dentist. Regular check-ups and flosisng won't prevent 1 in 20 of the 1,521 workers directly exposed to phosphorus fumes from contracting phossy jaw. 1 in 5 of them will die of the disease.
The accepted lethal dose when white phosphorus is ingested orally is 1 mg/kg, although the ingestion of as little as 15 mg has resulted in death. It may also cause liver, heart or kidney damage. There are reports of individuals with a history of oral ingestion who have passed phosphorus-laden stool ("smoking stool syndrome").
Phosphorus in rat poison was used by many to commit suicide, or even murder.
One theory is that it is caused by phosphorus in decaying flesh.

